Microsoft ends support for Internet Explorer on June 16, 2022.
We recommend using one of the browsers listed below.
- Microsoft Edge(Latest version)
- Mozilla Firefox(Latest version)
- Google Chrome(Latest version)
- Apple Safari(Latest version)
Please contact your browser provider for download and installation instructions.
February 12, 2019
Nippon Telegraph and Telephone Corporation
School of Science, The University of Tokyo
Creation of novel material Sr3OsO6 with the highest ferromagnetic transition temperature among insulators - Breaking the world record for the first time in 88 years -
Researchers of Nippon Telegraph and Telephone Corporation (NTT) have synthesized Sr3OsO6 , a novel material that exhibits ferromagnetism*1 above 780℃, which is the highest temperature among insulators. In collaboration with the Tsuneyuki's Research Group at the University of Tokyo (UTokyo), they have also revealed the electronic state*2 of this material, which is the key to comprehending the origin of the emergent ferromagnetism.
Our discovery surpasses the long-standing Curie temperature*3 (Tc) record among insulators for the first time in 88 years and is thus epoch-making for the development of magnetic materials. It also provides fundamental knowledge about the mechanism of the emergent ferromagnetism at high temperatures. Unlike most conventional magnetic materials, our brand-new material is free from Fe (iron) and Co (cobalt) and hence paves a new way to the exploration and development of other novel magnetic materials. Furthermore, the Sr3OsO6 was synthesized in the form of single-crystalline thin films.*4 This suggests that the Sr3OsO6 films can be readily implemented in device fabrication and are thus promising for high-performance magnetic devices that can be stably operated at high temperatures (room temperature to 250℃). Examples of such devices include magnetic random access memories (MRAM) and magnetic sensors.
This research was reported in Nature Communications on February 12, 2019.
1.Background
Ferromagnetic insulators*5 include maghemite, the first magnet that humans discovered and used as a compass. Today, ferromagnetic insulators are widely used as permanent magnets and in the microwave devices incorporated into, for instance, smartphones, cars, and computers--and such technology could not have been developed without ferromagnetic insulators. Recently, spintronic devices, in which both the electrical and magnetic properties of electrons are utilized simultaneously, are being intensely investigated to realize high-speed devices with low power consumption. Ferromagnetic insulators are also thought to be essential constituents that will make such spintronic devices viable.
In conjunction with trends in computerization, there has been a steadily growing demand for practical devices with higher performance. In terms of temperature, stable operation even above 200℃ is required. However, the record Tc, which is the crucial factor determining the temperature range in which any ferri/ferromagnetic system remains stable, has stood in insulators ever since ferrite magnets*6 were first developed over eight decades ago in the 1930s. Therefore, researchers have sought to develop the next generation of ferromagnetic insulators with high Tc's as well as establish guiding principles to search for such materials.
2.Achievements
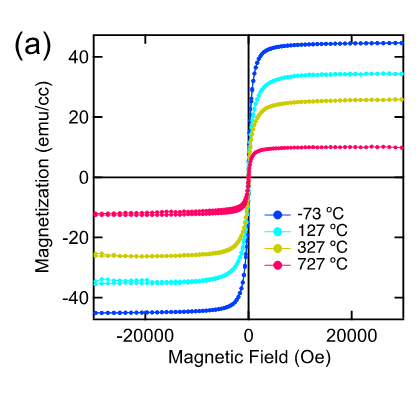
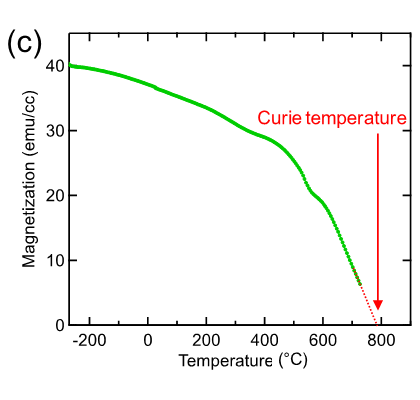
Researchers of NTT Basic Research Laboratories (NTT-BRL) have synthesized a novel material, Sr3OsO6, (Fig. 1) using a unique oxide thin-film growth technique that they have developed over many years. The Tc value of this material, estimated from the magnetic measurements, is above 780℃ (Fig. 2), which surpasses the Tc record among insulators for the first time in 88 years by more than 100℃.
Density functional theory*7 calculations carried out by the UTokyo team revealed that the ferromagnetic insulating state of Sr3OsO6 originates from the large spin-orbit coupling*8 of the 5d element*9 Os. This insight into the mechanism of the emergent high-temperature ferromagnetism will open a new avenue for developing functional materials in which elements having large spin-orbit coupling play a role.
Sr3OsO6 was synthesized in the form of single-crystalline thin films, which have high compatibility with device fabrication processes. This is in marked contrast to typical new oxides often synthesized in a powder or sintered polycrystalline form. Thus, Sr3OsO6 is expected to be readily implemented in high-performance magnetic device applications, such as magnetoresistive random access memories (MRAM) and magnetic sensors that work above room temperature.
3.Technical Features
Preparation of the high-quality Sr3OsO6 thin films
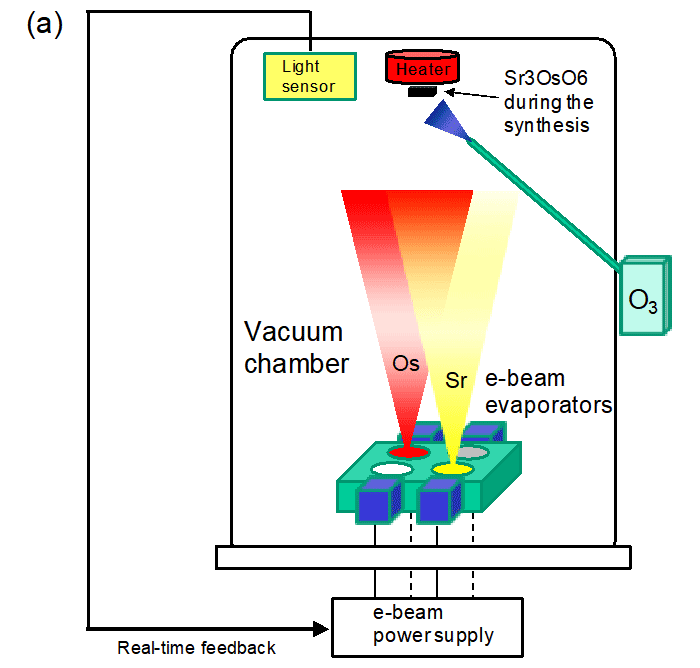
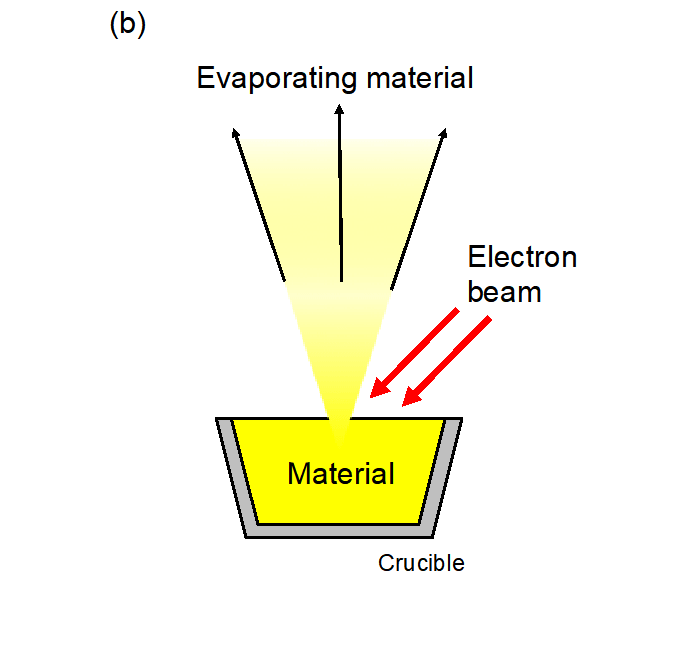
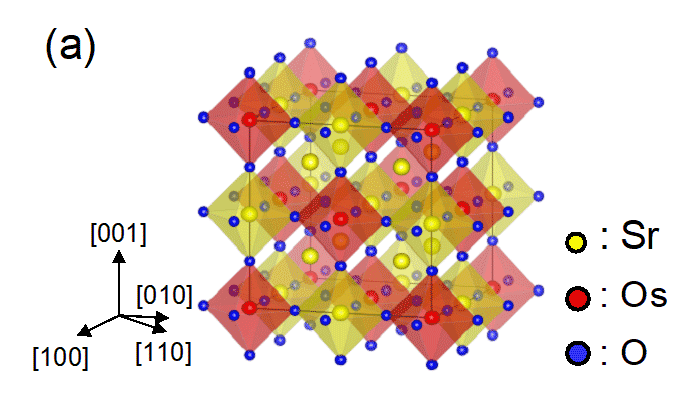
We used the molecular beam epitaxy*10 method to synthesize the Sr3OsO6 thin films, which have a crystal structure called double perovskite*11 (Fig. 1a). To grow high-quality Sr3OsO6 thin films, precise control of the flux rate of each constituent cation (Os, Sr) is mandatory. Generally, controlling the flux of Os is a challenge because of its high melting point (3033℃). Nevertheless, we have succeeded in controlling both the Sr and Os flux rates precisely. We accomplished this by monitoring the flux rates with an atomic emission spectrometer and feeding them back to the evaporation source power supplies in real time (Fig. 3), which enabled the synthesis of Sr3OsO6 thin films with the Sr and Os atoms arranged in a highly ordered structure (Fig. 1b).
4.Future plans
In our quest to better understand the fundamentals of ferromagnetism, we will further investigate the electronic structures of Sr3OsO6 using advanced spectroscopy techniques provided by synchrotron radiation facilities*12. Toward the development of high-performance magnetic devices that can be operated at high temperatures, we are trying to fabricate some test devices comprising Sr3OsO6 to examine the tunnel magnetoresistance effect.*13
4.Future plans
- Ferromagnetism
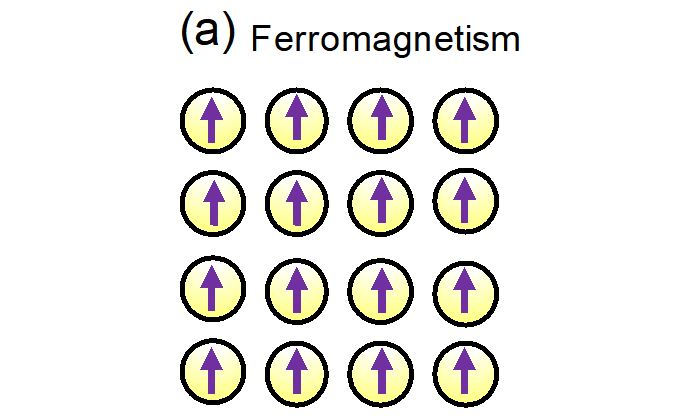
Ferromagnetism is a magnetic state in a material with which it can behave as a magnet. In the ferromagnetic state, the net magnetic moment is large since the magnetic moments of the constituent atoms are aligned (Fig. 4a). - Electronic state
Electrons in solids take certain states inherent to each material due to material-dependent electron-nuclei interactions, electron-electron interactions, and so on, unlike free electrons moving in a vacuum without such interactions. Such states in solids are called electronic states. Since the electronic states determine the properties of materials, elucidating them is an important step in materials science research and development. - Curie temperature
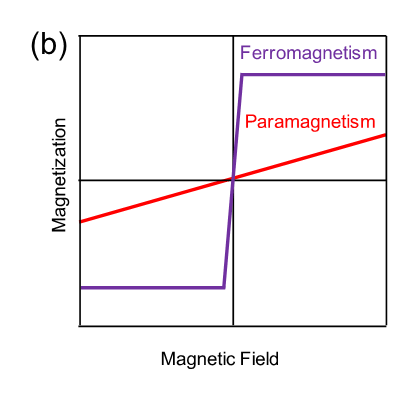
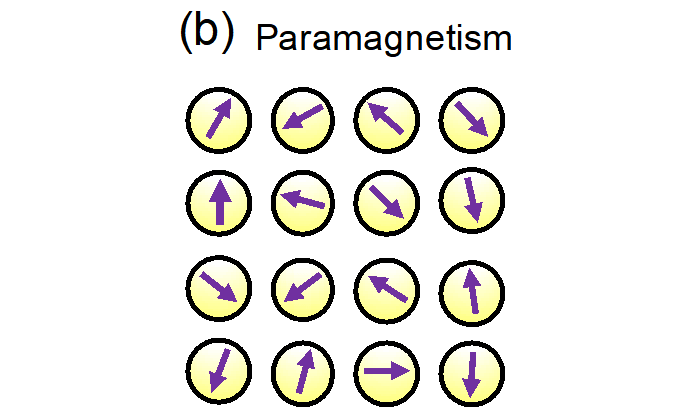
The Curie temperature is the temperature above which ferromagnetism in a magnet disappears. Below the Curie temperature, a magnet has a large magnetic moment, since magnetic moments of the constituent atoms are aligned (Fig. 4a). Above the Curie temperature, a magnet does not have a net magnetic moment and shows paramagnetism, since magnetic moments of the constituent atoms are not aligned but random (Fig. 4b). - Single-crystalline thin film
Solids in which atoms are periodically and orderly arranged and thus form lattices are called crystals. Crystals that have only a single atomic arrangement over an entire volume are called single crystals. Samples whose thicknesses range from one atomic layer to about several tens of micrometers (1μm = 1×10-6m) are called thin films. Single-crystalline thin films are synthesized on single-crystalline substrates. In this study, single-crystalline Sr3OsO6 thin films with the thickness of 300nm (1nm = 1×10-9m) were synthesized on single-crystalline SrTiO3 substrates. For microfabrication of high-performance devices, it is necessary to prepare samples in the form of single-crystalline thin films with submicrometer thicknesses. - Ferromagnetic insulator
A ferromagnetic insulator is a magnet in which electric current cannot flow due to its high resistivity. - Ferrite magnets
Ferrite magnets are ferromagnetic insulators developed in the 1930s in Japan. They have been the most widely used magnets in the world. Their major ingredients are iron oxides, and many ferrite magnets also contain Co (cobalt), Ni (nickel), Mn (manganese), and other elements. - Density functional theory
According to density functional theory, the energies of electrons in solids can be determined by using spatially dependent electron density n(r). The word "functional", which means the function of another function, is used since the energy is calculated as a function of n(r), which is already a function of r. Based on this theory, electronic states of materials can be calculated and predicted from the fundamental equation that electrons follow, without experimental data. - Spin-orbit coupling
Spin-orbit coupling is often compared with astronomical rotation and revolution. The spin magnetic moment coming from the axial rotation of electrons interacts with the orbital magnetic moment coming from the revolution of the charged particles (electrons) around the nucleus (Fig. 6). The influence of spin-orbit coupling on the electronic structures in Fe (iron), Co (cobalt), and ferrite magnets (mainly composed of iron oxides) is small. In sharp contrast, in Sr3OsO6, the large spin-orbit coupling of the 5d element*9 Os (osmium) plays an important role. - 5d element
The elements in groups 3-11 of the periodic table are called transition elements. The transition elements in the sixth period (row) are 5d elements (Fig. 5). The 5d elements include some noble metals, e.g., Au (gold) and Pt (platinum). Since a heavier element in the lower row of the periodic table has a larger spin-orbit coupling*8, the spin-orbit coupling in Os is larger than in Fe and Co. Os is used for the nib of fountain pens in the form of Os-Ru (ruthenium)-Ir (iridium) alloys. - Molecular beam epitaxy
Molecular beam epitaxy (MBE) is a method for growing high-quality single-crystalline thin films. In MBE, thin films crystallize via reactions between molecular or atomic beams of the constituent elements on heated single-crystal substrates (Fig. 3). Generally, this method is used to prepare single-crystalline thin films of existing materials to make superlattices, junctions, and devices. In this study, we synthesized the brand-new material (Sr3OsO6) using this method. - Double perovskite
In crystals, atoms are regularly ordered and form lattices. There are many kinds of atomic arrangements (crystal structures) and representative ones have specific names. One such arrangement is double perovskite, whose lattice is twice as large as the perovskite structure. It is widely known that many complex oxides take the perovskite structure. Recently, iodides and chlorides that take the perovskite structure have also been intensively studied for the next generation of solar cells. - Synchrotron radiation facilities
In synchrotron radiation facilities, we can use lights with various wavelengths (synchrotron radiation) such as ultraviolet rays and X-rays. Synchrotron radiation is emitted from accelerated electrons that travel in a huge ring in an ultrahigh-vacuum environment. The versatile capabilities of such synchrotron facilities include emissions of intense and variable-wavelength lights, which enable us to perform many kinds of high-resolution spectroscopies to investigate the physical properties of specimens in detail. Therefore, synchrotron radiation facilities are also very useful for materials science research. In Japan, there are several synchrotron facilities, including SPring-8 in Hyogo Pref. and the Photon Factory in Ibaraki Pref. - Tunnel magnetoresistance effect
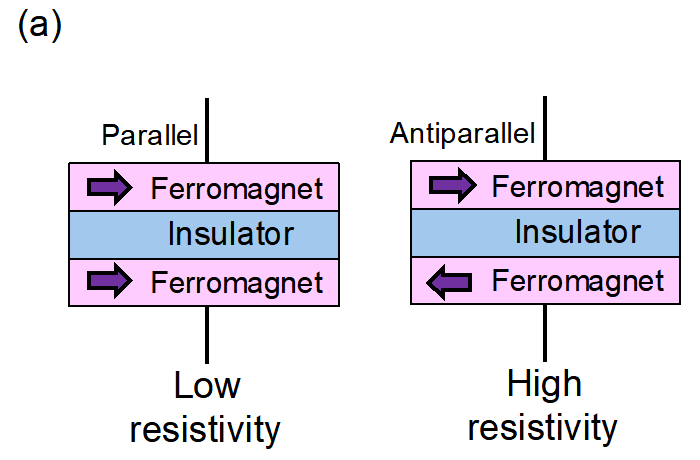
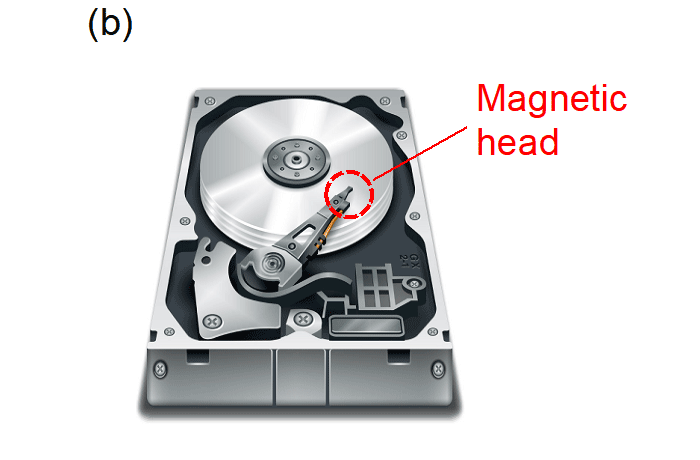
Tunnel magnetoresistance effect is that the tunnel resistance of an insulator sandwiched between two ferromagnets changes depending on the magnetic configuration of the ferromagnets (parallel or antiparallel) (Fig. 7a). The tunnel magnetoresistance effect has been widely utilized in commercial devices such as hard disc drives (HDDs), magnetoresistive random access memories (MRAMs), and magnetic sensors (Fig. 7b).
>Fig. 1a: Schematic diagram of Sr3OsO6 (double perovskite). The yellow, red, and blue spheres indicate Sr, Os, and O atoms, respectively. Fig. 1b: Atomic scale microscopy (scanning transmission electron microscopy) image of a Sr3OsO6 film viewed along the [110] direction. We can clearly see the atomic ordering depicted in Fig. 1a.
Fig. 2a: The magnetization versus applied magnetic field curves of a Sr3OsO6 film, showing ferromagnetic behavior with a finite magnetization even at the high temperature of 727℃. Fig. 2b: Schematic diagram of the magnetization versus applied magnetic field curve for ferromagnetism and paramagnetism. Fig. 2c: The magnetization versus temperature curve of a Sr3OsO6 film. The applied magnetic field is 2000 Oe. The Tc value, at which ferromagnetism disappears, is above 780℃.
Fig. 3a: Schematic diagram of the molecular beam epitaxy system used in this study. We control the elemental fluxes of both Sr and Os by feeding the measured flux rates back to the power supply of the e-beam evaporators in real time. The flux rates are measured with a light sensor that detects specific wavelength lights emitted from the evaporant fluxes. Fig. 3b: Schematic illustration of the e-beam evaporation. Electron beam irradiation increases temperatures of the source materials. A larger e-beam current gives a larger flux rate.
Fig. 4a: Schematic diagram of ferromagnetism. Fig. 4b: Schematic diagram of paramagnetism. The purple arrows indicate the magnetization of each atom.
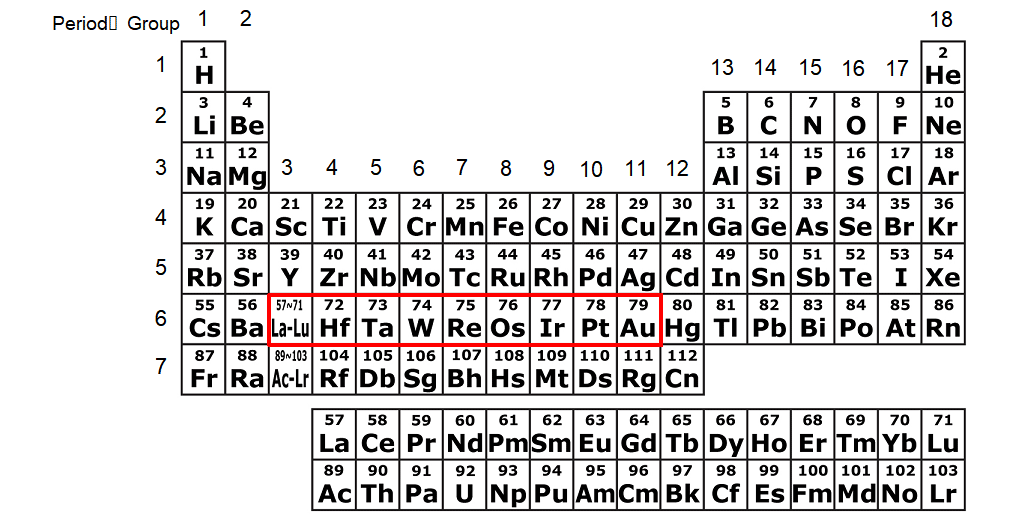
Fig. 5: The periodic table. The elements enclosed by the red frame are called 5d transition elements
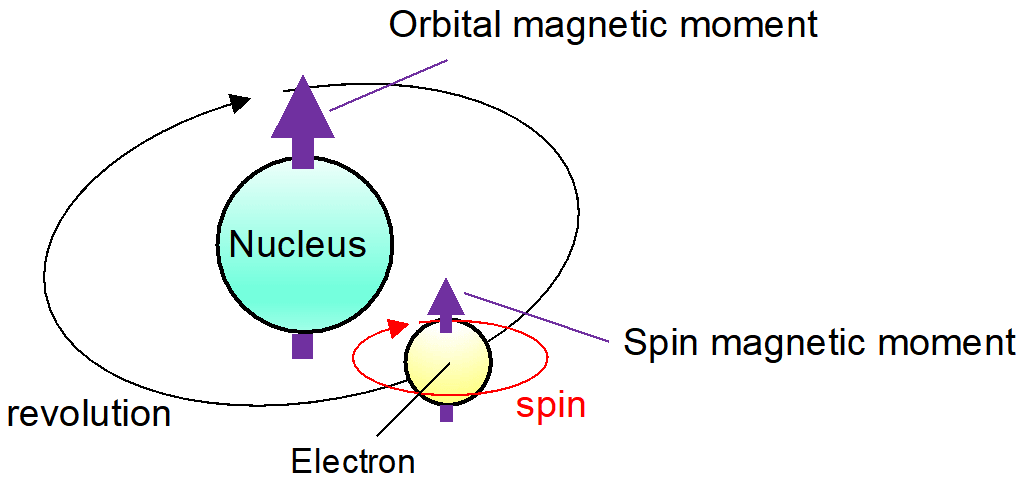
Fig. 6: The spin magnetic moment coming from the rotation of the electron, and the orbital magnetic moment coming from the revolution of the charged particle (electron) around the nucleus.
Fig. 7a: Tunnel magnetoresistance effect. The resistivity of the insulator sandwiched between the two ferromagnets becomes low when the magnetic configuration of the ferromagnets is parallel and vice versa. Fig. 7b: The tunnel magnetoresistance effect has been used for the magnetic head in hard disc drives (HDDs).
Press contact
Nippon Telegraph and Telephone Corporation
Science and Core Technology Laboratory Group, Public Relations
science_coretech-pr-ml@hco.ntt.co.jp
TEL: +81-46-240-5157
The University of Tokyo
The Office of Communication, Graduate School of Science
kouhou.s@gs.mail.u-tokyo.ac.jp
TEL: +81-3-5841-8737
Information is current as of the date of issue of the individual press release.
Please be advised that information may be outdated after that point.
NTT STORY
WEB media that thinks about the future with NTT



![Fig. 1b: Atomic scale microscopy (scanning transmission electron microscopy) image of a Sr3OsO6 film viewed along the [110] direction.](img/190212ab.gif)