Microsoft ends support for Internet Explorer on June 16, 2022.
We recommend using one of the browsers listed below.
- Microsoft Edge(Latest version)
- Mozilla Firefox(Latest version)
- Google Chrome(Latest version)
- Apple Safari(Latest version)
Please contact your browser provider for download and installation instructions.
February 6, 2023
NTT Corporation
Shizuoka University
Japan Science and Technology Agency (JST)
Detection of ferric ions in neurons using a superconducting flux qubit
- Towards pathology examinations at the single-cell level -
NTT Corporation (NTT, Head office: Chiyoda-ku Tokyo; President & CEO: Akira Shimada) and Shizuoka University (Shizuoka-shi, Shizuoka; President: Kazuyuki Hizume) have devised a way to detect ferric ions in single neurons by using a superconducting flux qubit1.
In this work, ferric ions in neurons were detected and quantified using a superconducting flux qubit with a size around ten micrometers. Although this proof of principle experiment used a single qubit, the single qubit can be replaced with a qubit array to image distributions of ions.
The application area of this technique can be extended to various biomaterials. This will enable pathological examinations with single-cell resolution.
This research was reported in the British journal, Communications Physics on February 6, 2023.
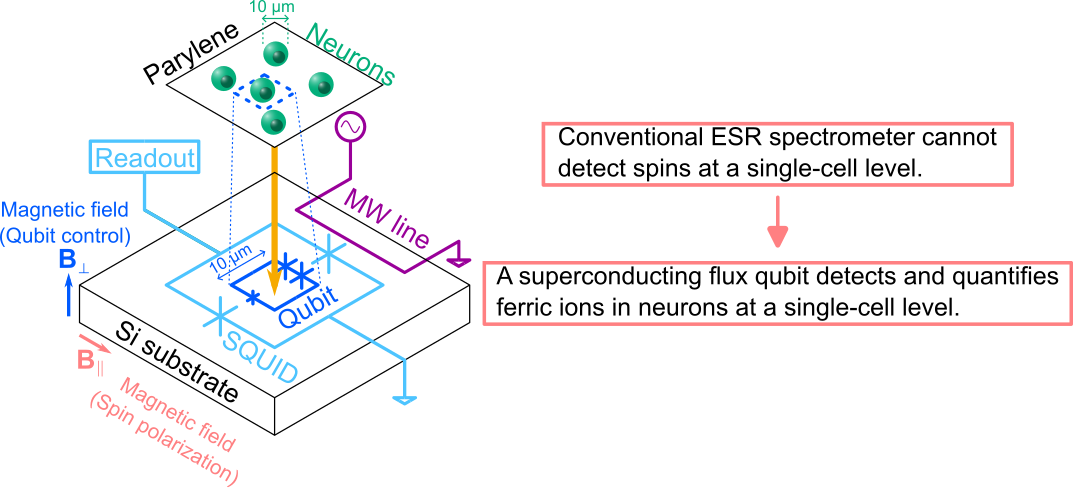 Fig. 1 Overview of this study.
Fig. 1 Overview of this study.
1.Background
Iron is a most abundant trace element in the human body and plays important physiologic and pathologic roles. One of the most frequently used methods to investigate such metallic elements is electron spin resonance (ESR)2. The ESR spectrum gives fruitful information about trace elements like redox states. However, a conventional spectrometer requires several milliliters of sample, containing more than 1013 spins. This feature prevents the investigation of trace elements at a single-cell level or imaging of the distribution of metallic ions.
The research group had previously identified impurities in solid-state materials by using a superconducting flux qubit. This method features a high sensitivity of 20 spins/√Hz and high spatial resolution of a few micrometers. It is also possible to apply it to biosensing, in which trace elements in biological tissues are analyzed, at a single-cell level.
2.Achievements
The research group measured the magnetization3 from neurons by using a highly sensitive magnetometer based on a superconducting flux qubit (Fig. 1). Combining magnetometry and conventional ESR spectral measurements, the origin of the magnetization was identified as ferric ions in the neurons. The magnetometry also gave quantitative information: the dried neurons contained iron with a concentration of 6 μg/g, a value that is consistent with the literature reports.
This result can be extended to a broad range of biomaterials. By imaging trace elements at the single-cell level, more detailed information can be obtained in pathological examinations.
NTT designed the experiment, prepared the neurons, and measured the superconducting qubit. Shizuoka University performed ESR spectroscopy.
3. Technical features
(1) A superconducting flux qubit is used as a magnetometer with high sensitivity and high spatial resolution. The qubit can detect 20 electron spins (ferric ions), and the detection area is typically several to 100 μm2, as determined by the loop area of the flux qubit.
(2) Neurons are cultured on a biocompatible insulation film, parylene4. The thickness of the film should be minimized to keep high magnetic sensitivity. In this study, the thickness was 2 μm (Fig. 4).
4. Outlook
The reported method enables magnetometry of biological samples at the single-cell level with quantitative information. A conventional ESR spectrometer5 is currently used to identify the ion species. In future, the method will be improved to perform ESR spectroscopy on chip.
The sensitivity and spatial resolution will be further improved towards single spin detection. The development of the sensing schemes with high sensitivity and high spatial resolution may lead to the realization of advanced pathology examinations that will enable advanced treatments of diseases.
[Technical details]
[1] Magnetometry using a superconducting flux qubit
The superconducting flux qubit used in this study is one of several kinds of superconducting qubit. A flux qubit is suitable for magnetometry since its sensitivity to a magnetic field can be enhanced by changing the design parameters. The sample for magnetometry was prepared by culturing neurons on parylene film and bonding the film to the qubit chip. Parylene was used as an insulation film to electrically isolate the qubit from the neurons.
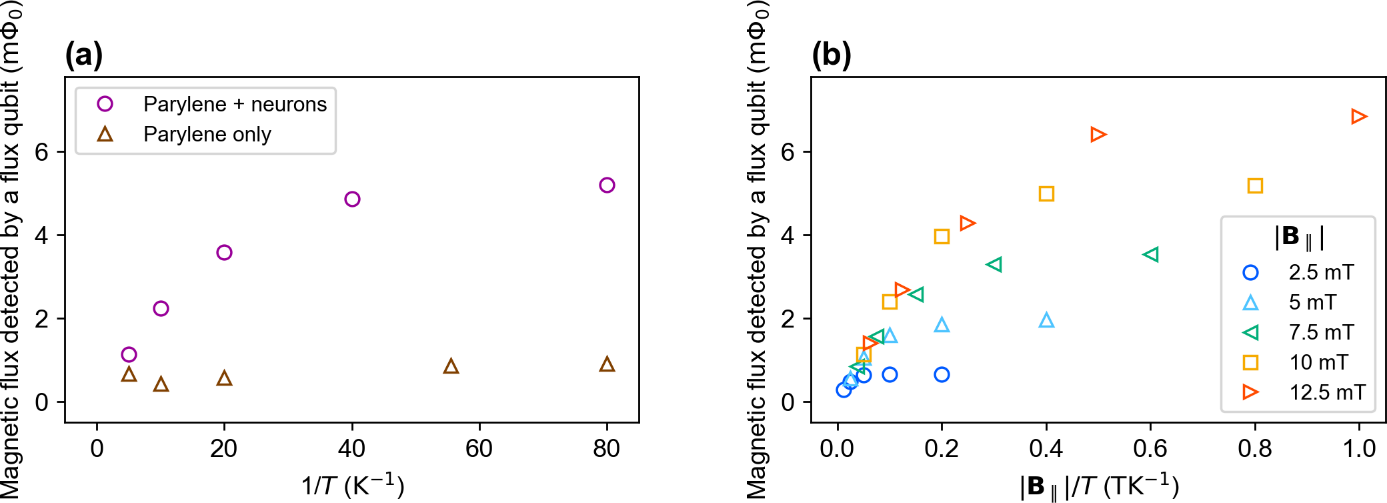 Fig. 2 (a) Temperature dependence of the magnetic flux detected by a flux qubit under a magnetic field of 10 mT. (b) Temperature dependence of the magnetic flux detected by a flux qubit under various magnetic fields.
Fig. 2 (a) Temperature dependence of the magnetic flux detected by a flux qubit under a magnetic field of 10 mT. (b) Temperature dependence of the magnetic flux detected by a flux qubit under various magnetic fields.
The magnetization of the sample originates from the alignment of electron spins. By applying a magnetic field to a sample, unpaired electrons6 align at low temperatures, resulting in a large magnetization. On the other hand, unpaired electrons are randomly oriented at high temperatures, resulting in a small magnetization. This phenomenon was confirmed by magnetometry using a superconducting flux qubit. The circles in Fig. 2 (a) show the temperature dependence of the magnetization under a magnetic field of 10 mT. The magnetic flux detected by the superconducting flux qubit increased as the temperature decreased. Here, the magnetization of the sample was detected as magnetic flux. The magnetization of the sample also depends on the applied magnetic field, as investigated in Fig. 2 (b). The figure clearly shows that the magnetization increased as the applied field increased. These experimental results indicate that the sample contained unpaired electrons.
[2] Identification of the origin of the magnetization
To determine the origin of the magnetization, we performed magnetometry with different experimental configurations. The magnetization may have originated from the neurons, insulation film, or both. The magnetization of the insulation film itself was measured as a control [Fig. 2 (b), triangles]. Compared with the results for the sample with neurons, it can be seen that the contribution to the magnetization from the insulation film is negligible. This proves that the neurons mainly contributed to the magnetization.
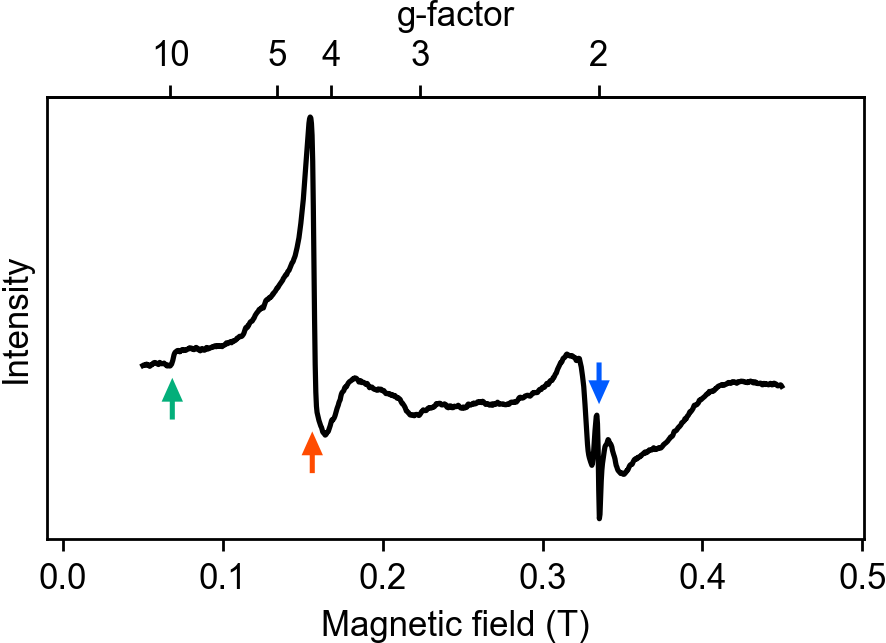 Fig. 3 ESR spectrum of neurons. The green (red, blue) arrow indicates the peak with g=9.8 (4.3, 2.0), respectively
Fig. 3 ESR spectrum of neurons. The green (red, blue) arrow indicates the peak with g=9.8 (4.3, 2.0), respectively
Neurons may contain several different trace elements. However, magnetometry cannot distinguish the type. To identify the type of trace element, the ESR spectrum was obtained using a conventional spectrometer. The spectrum in Fig. 3 shows three peaks with g-factors7 of 9.8, 4.3, and 2.0. The first two peaks are specific to ferric ions. The intensity of the peak with g = 9.8 dominates the spectrum, which indicates that the magnetization originated from the ferric ions in the neurons. The small peak with g = 2.0 may have originated from other ions, like copper.
[3] Quantitative analysis of ferric ions
The measured magnetization has a direct relationship with the number of electrons in the sample. Thus, the number of iron atoms in the sample can be estimated. The conversion factor from magnetization to the number of spins is known from our previous experiment. Combining this value with the detection volume of the superconducting flux qubit, the mass density of iron atoms in dry neurons was estimated to be 6 μg/g, which is consistent with the literature. It should be noted that single or a few neurons are contained in the detection volume of the superconducting flux qubit. This result clearly shows that quantitative analysis of trace elements in such a small number of cells is possible.
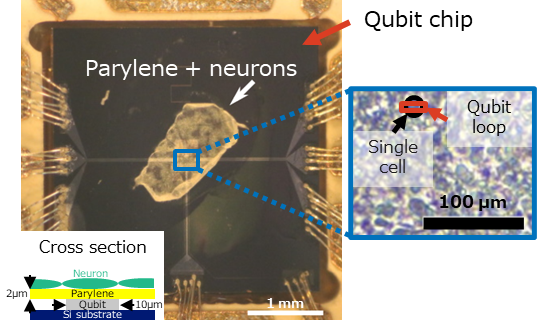 Fig. 4 Neurons cultured on a parylene film.
Fig. 4 Neurons cultured on a parylene film.
Support for this research
Some of the results of this research were obtained with the support of the Japan Science and Technology Agency (JST) CREST (JPMJCR1774).
Publication information
Journal: Communications Physics
Title: "Magnetometry of neurons using a superconducting qubit"
Authors: Hiraku Toida, Koji Sakai, Tetsuhiko F. Teshima, Masahiro Hori, Kosuke Kakuyanagi, Imran Mahboob, Yukinori Ono, and Shiro Saito
<Glossary>
1.Superconducting flux qubit
A superconducting flux qubit is a type of qubit consisting of a superconducting loop and Josephson junctions. The property of the qubit can be tuned by varying the magnetic field penetrating the loop. The magnetic field generated by ferric ions in neurons can modulate the properties of the qubit, causing it to function as a magnetometer for neurons.
2.Electron spin resonance
Magnetic resonance imaging (MRI) is a commonly used technique in medical diagnosis. This method visualizes the properties of nuclei in the body. On the other hand, electron spin resonance is a method to characterize the electron spins in a sample.
3.Magnetization
In materials under a magnetic field, electron spins align in one direction. In this case, the materials have properties like a magnet.
4.Parylene
Parylene is a polymer with a linear crystalline structure that is a good insulator and acts as a strong barrier against moisture and chemical substances. In addition, it has excellent heat resistance and ultraviolet resistance and is highly biocompatible. It is used as a coating agent in a wide range of industrial fields including medical care.
5.Electron spin resonance spectrometer
A conventional electron spin resonance spectrometer, which is used to analyze materials, consists of a microwave cavity (metal box), a measurement system of microwave transmittance, and strong electromagnets. The sample is inserted into the cavity. The microwave transmittance is measured as a function of a magnetic field by applying microwaves resonant with the cavity. The energy splitting of the electron spin increases with the magnetic field strength. If the microwave energy resonates with the energy splitting of the electron spin, peaks should appear in the microwave transmittance. The peaks contain the information about the electron spins. Thus, material properties can be determined by analyzing the position (a qualitative figure) and height (a quantitative figure) of the peak.
6.Unpaired electron
These are electrons in materials which do not form pairs. Materials containing unpaired electrons are chemically active and have magnetic properties. Such materials can be investigated using electron spin resonance spectrometers.
7.g-factor
One of the parameters obtained from the ESR spectrum. Without any interaction with the environment, an electron has a g-factor of 2.0023193. However, the g-factor may be modified in materials due to interactions with other electrons or nuclei. The value reflects the properties of the unpaired electrons in the materials. Thus, the g-factor can be used as a fingerprint of materials.
Contact Information
NTT Corporation
Science and Core Technology Laboratory Group, Public Relations
nttrd-pr@ml.ntt.com
Shizuoka University
General Affairs Division, Public Relations and Funding Section
koho_all@adb.shizuoka.ac.jp
About the CREST Program
Japan Science and Technology Agency
Department of Strategic Basic Research,
Yuko Shimabayashi
crest@jst.go.jp
Information is current as of the date of issue of the individual press release.
Please be advised that information may be outdated after that point.
NTT STORY
WEB media that thinks about the future with NTT