Microsoft ends support for Internet Explorer on June 16, 2022.
We recommend using one of the browsers listed below.
- Microsoft Edge(Latest version)
- Mozilla Firefox(Latest version)
- Google Chrome(Latest version)
- Apple Safari(Latest version)
Please contact your browser provider for download and installation instructions.
October 27, 2023
NTT Corporation
World's Longest Carbon Fixation in Artificial Photosynthesis Using Semiconductor Photocatalysts
Achieving carbon fixation exceeding the annual carbon fixed by trees for 350 hours of continuous operation
Tokyo - October 27, 2023 - NTT Corporation (NTT) has developed an artificial photosynthetic device that combines a semiconductor photocatalyst that uses solar energy and a metal catalyst that reduces carbon dioxide (CO2) as an electrode to achieve the world's longest continuous carbon fixation for 350 hours. The cumulative amount of carbon fixed by the conversion of CO2 reaches 420 g/m2, which is more than the amount of carbon per fixed unit area of Cryptomeria japonica plant per year. In the future, we will strive to achieve both higher efficiency and longer life for electrodes to realize higher performance artificial photosynthesis reactions. Through outdoor testing, we will contribute to the mitigation of climate change and the realization of a sustainable society as one of the technologies to reduce CO2 using solar energy. The results will be displayed at the NTT R&D Forum IOWN ACCELERATION [1], which will be held from November 14th to 17th, 2023.
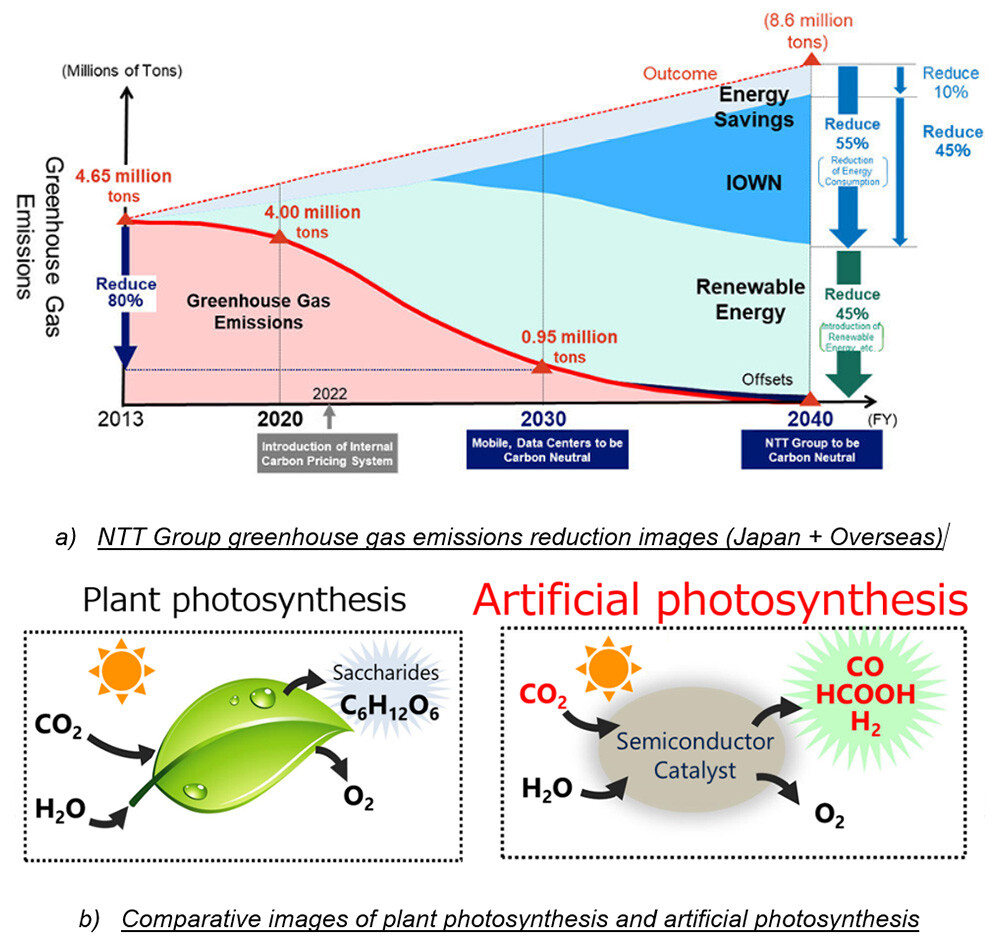 Figure 1 NTT's Carbon Neutrality and Artificial Photosynthesis Research and Development
Figure 1 NTT's Carbon Neutrality and Artificial Photosynthesis Research and Development
1. Background
Decarbonization efforts are accelerating around the world to curb climate change. To achieve carbon neutrality within the NTT Group in 2040 [i], the NTT Group is implementing various initiatives to reduce greenhouse gas emissions, including introducing renewable energy and reducing power consumption through IOWN (Figure 1, a). In addition to these technologies, we are conducting research and development on artificial photosynthesis, which is composed of inorganic materials such as semiconductors [2] and catalysts [3] and can achieve a conversion performance of CO2 [4] exceeding that of plant photosynthesis, as a technology for converting and immobilizing CO2 already emitted or emitted into the atmosphere into carbon monoxide (CO) [5] and formic acid (HCOOH) [6] (Figure 1, b).
Various studies on artificial photosynthesis have been carried out around the world, particularly on catalysts that can achieve high CO2 conversion efficiency [7]. On the other hand, the test time for continuous CO2 conversion remains at the level of several hours to several tens of hours, making it an issue to establish the technology to control degradation for longer time.
2. Research Results
As shown in Figure 2, artificial photosynthesis consists of an oxidation electrode [8] using a semiconductor photocatalyst [9] and a reduction electrode [10] using a metal catalyst. As a concrete issue for practical application, it is required to design electrodes with a long life that can resist degradation due to corrosion and can withstand reactions for a long time. The conversion of CO2 by artificial photosynthesis is widely used by reducing dissolved CO2 in an aqueous solution to CO and HCOOH. However, the amount of CO2 that can be dissolved in an aqueous solution is limited, and side reactions [11] are likely to occur. Therefore, electrode structures and device designs that selectively convert CO2 are required.
NTT has designed an artificial photosynthesis device that consists of a long-life semiconductor photocatalyst electrode that uses light as energy and a fibrous metal catalyst electrode integrated with an electrolyte membrane to efficiently convert CO2 in the gas phase.
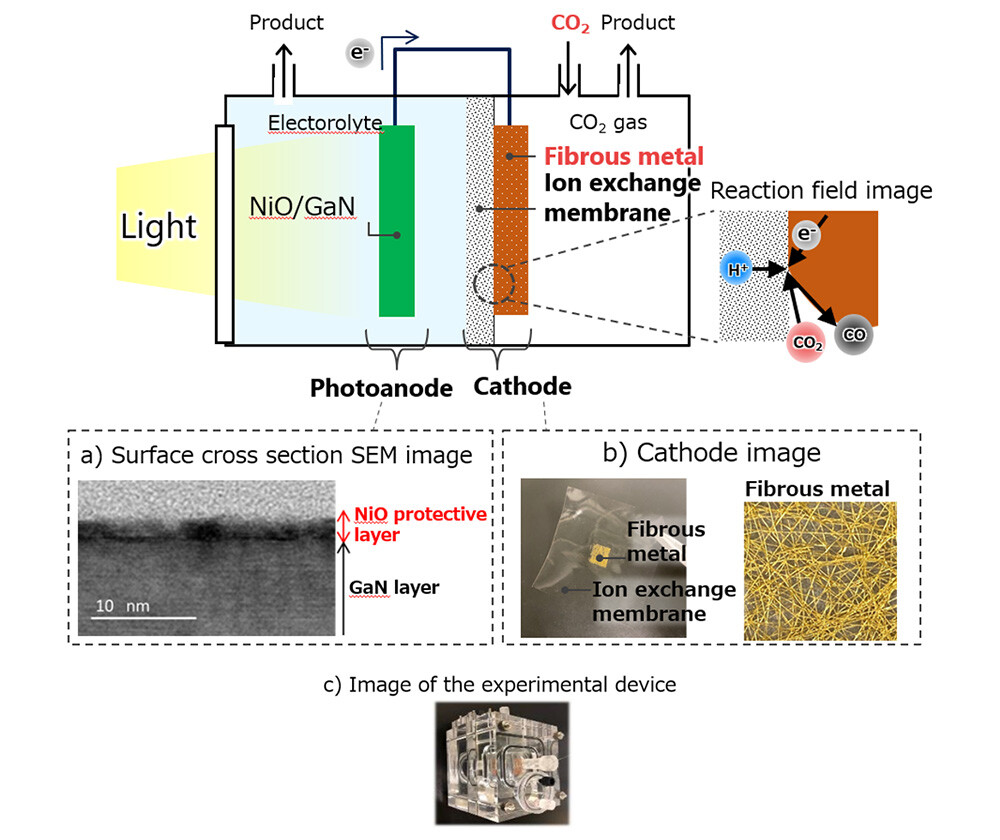 Figure 2 Schematic Diagram of the Artificial Photosynthesis Device
Figure 2 Schematic Diagram of the Artificial Photosynthesis Device
< Technology Points >
- Technology for suppressing degradation reaction of semiconductor photocatalyst electrode
For gallium nitride (GaN) [12] -based electrodes used as semiconductor photocatalysts, the challenge was to suppress degradation reactions occurring at the interface between the GaN surface and the aqueous solution. Therefore, by forming a uniform nickel oxide (NiO) [13] thin film with a thickness of 2 nm, which smooths the irregularities of the GaN surface and transmits light well, as a protective layer, the contact between GaN and aqueous solution was prevented (Figure 2, a), and the degradation of the electrode was greatly suppressed. - Gas phase CO2 conversion technology
The conventional metal electrode for converting dissolved CO2 has a plate-like structure. In order to convert CO2 in the gas phase, we have devised an electrode structure that integrates a fibrous metal with high diffusivity of CO2 and an electrolyte membrane [14] that supplies protons (H+) necessary for the CO2 conversion reaction to the reaction field (Figure 2b, Left). As a result, the proton (H+) required for the CO2 conversion reaction can be supplied to the reaction field without dipping the electrode in the aqueous solution, making it possible to directly convert CO2 in the gas phase. Thanks to these improvements in the electrode structure, the conversion efficiency of CO2 is more than 10 times higher than before.
The artificial photosynthesis device described above was irradiated with simulated sunlight [15]. As a result of the CO2 conversion test in the gas phase, it was confirmed that CO2 was converted to CO and HCOOH continuously for 350 hours. The cumulative amount of carbon fixed per unit area [16] calculated from generated CO and HCOOH reached 420 g/m2, making it the world's longest continuous operation for 350 hours in artificial photosynthesis using semiconductor photocatalysts.
The amount of carbon fixation from this verification is equivalent to more CO2 than a single tree (Cryptomeria japonica plant) fixes per square meter in about one year.
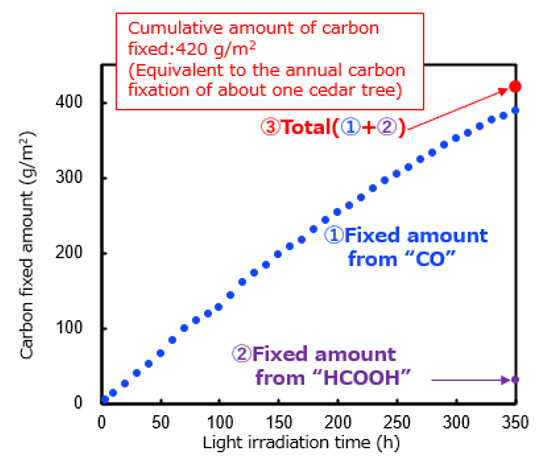 Figure 3 Change in the Amount of Fixed Carbon with Respect to the Light Exposure Time
Figure 3 Change in the Amount of Fixed Carbon with Respect to the Light Exposure Time
3. Outlook
To realize higher performance artificial photosynthetic reactions in the future, we aim to further increase the efficiency and longevity of semiconductor photocatalyst electrodes. In addition, we have established this technology as one of the technologies for reducing CO2 using solar energy through outdoor testing as well as laboratory testing, thereby contributing to the control of climate change and the realization of a sustainable society.
< Reference >
[i]NTT Group's New Environment and Energy Vision "NTT Green Innovation toward 2040"
https://group.ntt/en/newsrelease/2021/09/28/210928a.html
< Glossary >
[1]NTT R&D FORUM 2023 -IOWN ACCELERATION Official Website
https://www.rd.ntt/e/forum/2023/
[2]Semiconductors: Materials with resistivity intermediate between conductors such as metals with good electrical conductivity and insulators with high electrical resistivity.
[3]Catalyst: A substance that promotes the desired chemical reaction without changing the catalyst material itself.
[4]CO2 conversion performance: Ability to convert CO2 through photosynthesis
[5]Carbon monoxide: A gaseous substance at ambient temperature produced by the reduction of CO2 by electrons (e-) and H+.
[6]Formic acid (HCOOH): A liquid substance at room temperature produced by the reduction of CO2 by electrons (e-) and H+.
[7]CO2 conversion efficiency: Percentage of input solar energy used for CO2 conversion
[8]Oxidation electrodes: Electrodes that undergo water oxidation reactions (2H2O + 4h+ → O2 + 4H+) with light energy.
[9]Semiconductor photocatalyst: A semiconductor that does not change itself and promotes a chemical reaction by irradiating the semiconductor with light.
[10]Reduction electrode: Electrode where reduction reaction of CO2 occurs (CO2 + 2e- + 2H+ → CO + H2O or HCOOH)
[11]Side reaction: A reaction different from the target reaction
[12]Gallium nitride (GaN): This is a nitride of gallium and is widely used as a semiconductor device. Absorbs light in the ultraviolet light region and converts light energy
[13]Nickel oxide (NiO) is an oxide of nickel (Ni), and is a material that aids in the desired reaction. Thin film is used so that GaN and water do not come into direct contact and light is transmitted.
[14]Electrolyte membrane: A membrane placed between two electrodes that allows only H+ to move.
[15]Pseudo-sunlight: Illumination having characteristics of wavelength distribution close to sunlight
[16]Amount of fixed carbon: The amount of CO2 converted into another substance.
About NTT
NTT contributes to a sustainable society through the power of innovation. We are a leading global technology company providing services to consumers and business as a mobile operator, infrastructure, networks, applications, and consulting provider. Our offerings include digital business consulting, managed application services, workplace and cloud solutions, data center and edge computing, all supported by our deep global industry expertise. We are over $95B in revenue and 330,000 employees, with $3.6B in annual R&D investments. Our operations span across 80+ countries and regions, allowing us to serve clients in over 190 of them. We serve over 75% of Fortune Global 100 companies, thousands of other enterprise and government clients and millions of consumers.
Media contact
NTT
Public Relations, NTT Science and Core Technology Laboratory Group
nttrd-pr@ml.ntt.com
Information is current as of the date of issue of the individual press release.
Please be advised that information may be outdated after that point.
NTT STORY
WEB media that thinks about the future with NTT